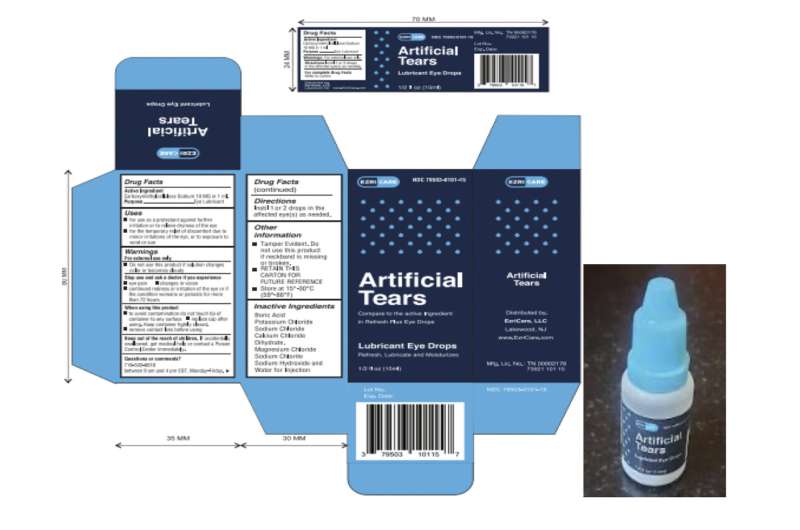
The producer of eyedrops not too long ago linked to deaths and accidents lacked measures to guarantee sterility at its manufacturing unit in India, in line with U.S. well being inspectors.
Meals and Drug Administration officers uncovered a couple of dozen issues with how International Pharma Healthcare made and examined its eyedrops throughout an inspection from late February by way of early March. The FDA launched its preliminary inspection report Monday.
The corporate makes use of procedures that may’t really guarantee its merchandise are sterile, FDA workers wrote. Particularly, the inspectors discovered that the plant had used “a poor manufacturing course of” between December 2020 and April 2022 for merchandise that had been later shipped to the U.S.
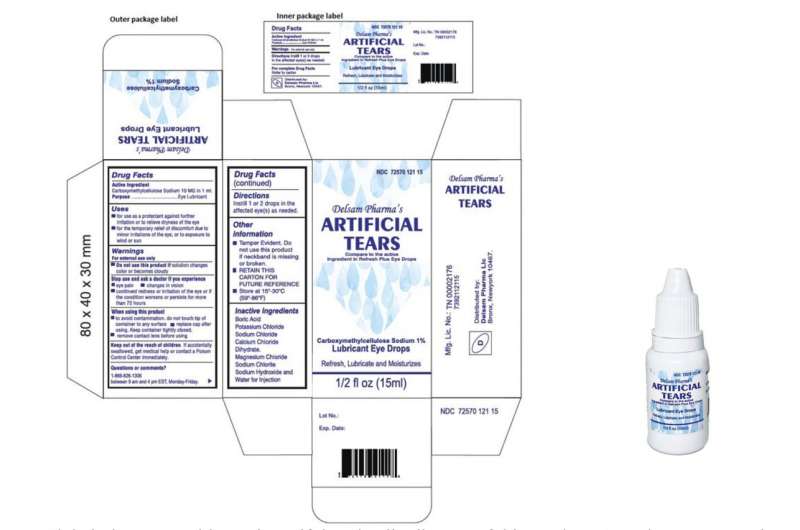
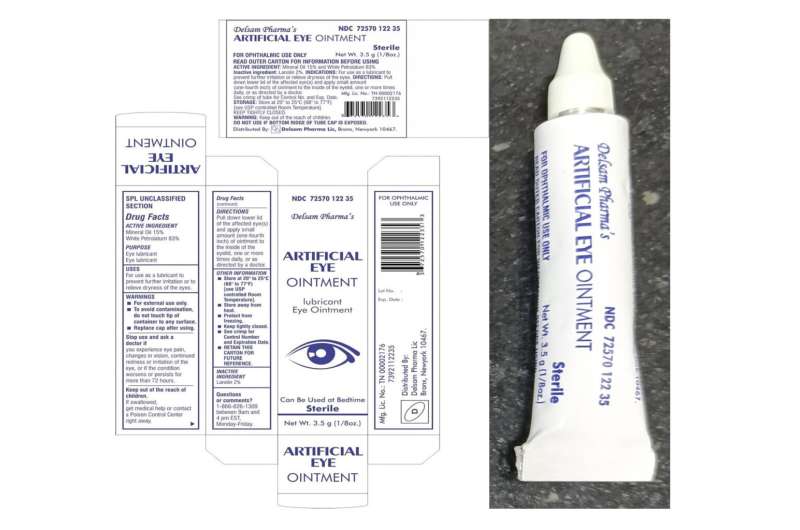
The plant in India’s southern Tamil Nadu state produced eyedrops which have been linked to 68 bacterial infections within the U.S., together with three deaths and eight instances of imaginative and prescient loss. 4 individuals have had their eyeballs surgically eliminated resulting from an infection. The drops had been recalled in February by two U.S. distributors, EzriCare and Delsam Phama.
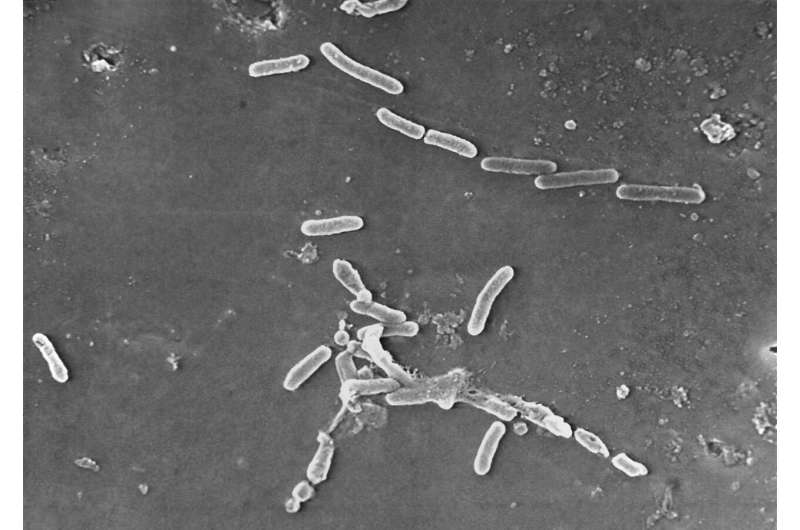
The outbreak is taken into account significantly worrisome as a result of the micro organism driving it’s resistant to straightforward antibiotics.
Inspectors arrived on the plant Feb. 20, greater than two weeks after the announcement of the primary eyedrop recall on Feb. 3. The inspection seems to be the FDA’s first go to to the plant, in line with company information.
The report has the company’s preliminary findings and is more likely to be adopted by a proper report and a warning letter to the corporate. An FDA spokesman mentioned the inspection signifies that the corporate’s merchandise “could also be in violation of FDA’s necessities.”
“We urge customers to cease utilizing these merchandise which can be dangerous to their well being,” FDA’s Jeremy Khan wrote in an emailed assertion.
The FDA is liable for assuring the security of overseas merchandise shipped to the U.S., although it has lengthy struggled to maintain tempo with worldwide pharmaceutical provide chains that more and more start in India and China.
FDA inspectors cited worrisome sanitary situations on the International Pharma plant, noting that its flooring, partitions and ceilings weren’t “simply washer-friendly.” At one level throughout the go to, an FDA inspector famous “not one of the tools on the filling machine was wrapped or coated.” The inspector additionally famous the corporate did not have rigorous procedures for guaranteeing bottles had been totally sealed. As a substitute, a “guide visible inspection is the one check to detect any leak,” in line with the report.
International Pharma has mentioned little publicly about its current remembers, as an alternative referring inquiries to the U.S. corporations that bought the merchandise.
The FDA has been investigating the U.S. bacterial infections alongside the Facilities for Illness Management and Prevention. CDC officers have detected the bacterial pressure in opened bottles of EzriCare drops collected from contaminated sufferers. FDA officers are additionally testing unopened bottles of the drops.
CDC officers are nervous the micro organism will unfold and instances could also be reported for weeks and months to return. The company has been urging well being care services treating sufferers to comply with strict infection-control suggestions as a result of the germ can unfold quickly.
© 2023 The Related Press. All rights reserved. This materials might not be printed, broadcast, rewritten or redistributed with out permission.
Quotation:
Eyedrops maker could not guarantee manufacturing unit was sterile, FDA says (2023, April 4)
retrieved 5 April 2023
from https://medicalxpress.com/information/2023-04-eyedrops-maker-couldnt-factory-sterile.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.









